- Lemur Project Downloads - June 2020. The Lemur Project (an NSF-funded collaboration with CMU and the CIIR) develops search engines, browser toolbars, text analysis tools, and data resources that support research and development of information retrieval and text mining software.
- We are here to help. If you have a specific inquiry or need assistance email info@amherstarea.com or call (413)253-0700 and the Chamber will connect you with the resources you need. Community Organizations Non-Profit Community Organizations Member Directory Arts, Culture, & Entertainment Education Employment Opportunities Career resources compiled with the help of the Western Massachusetts.
- Amherst Recognizing the exaggeration ways to get this book Principles of Management, MGT 301: University of Massachusetts-Amherst is additionally useful. You have remained in right site to start getting this info. Acquire the Principles of Management, MGT 301: University of Massachusetts-Amherst join that we pay for here and check out the link.
Solutions
UMass Amherst faculty, eligible staff, and GEO graduate students may install software available through the Adobe Campus Agreement on one personally-owned computer. To transfer your Adobe license to a new computer, you must first deactivate it from its original location. Uninstalling the software.
13.2 Boiling Point Elevation – Maple Syrup
Subjects: Solutions, Boiling point elevation, colligative properties
Description: Demonstrate that maple syrup has a higher boiling point than water. As maple syrup continues to boil, the solution becomes more and more concentrated as water is lost. The increasing concentration of sugar in the water increases the boiling point of the solution.
Materials:
- 100g grade A maple syrup‡ (or 67% w/w sugar solution)
- 400 ml beaker
- Stir bar
- Hot plate*
- Standalone thermometer or Vernier Stainless Steel Temp Probe, interface, and, Logger Pro or Logger lite software.
*Shared item: located in the top drawer of the center bench opposite the chemical storage shelves. The Temperature probes are located in the drawers opposite the bin storage shelves.
‡In refrigerator Motorcycle mechanics institute.
Procedure:
1. Heat the syrup in the beaker until boiling. Record the temperature.
2. Continue to stir and boil syrup.
3. Measure the temperature again over time. The temperature will increase as the water evaporates.
Discussion:
Maple sap has approximately 2% sugar. To produce maple syrup, the sap is boiled until enough water has evaporated to produce a syrup with approximately 67% sugar content with a boiling point of 219 degrees Fahrenheit (103.8˚ C). 1,2

Maple sap contains other solutes besides sugar, but assuming these concentrations are negligible, we can calculate the boiling point elevation of maple syrup compared with water.
Elevation in boiling point = ∆Tbp = Kbp*msolute
Maple sugar is primarily sucrose (C12H22O11) with a molecular weight of 342.30g/mol.
Assume we have 100 grams of Grade A light maple syrup containing 67% sugar. The solution is composed of 67 grams sucrose and 33 grams water. The Kbp for water is 0.5121˚C /m.
Moles of sucrose = 67g (1 mol/ 342.30 g) = 0.1957 mol sucrose
EA Sports Cricket 2007 PC Game Full Version Free Download Highly Compressed Zip File How to download Full Setup of EA Cricket 2007 For Windows XP/7/Vista/8/8.1/10 Pro Highly Compressed EA CRICKET 2007. 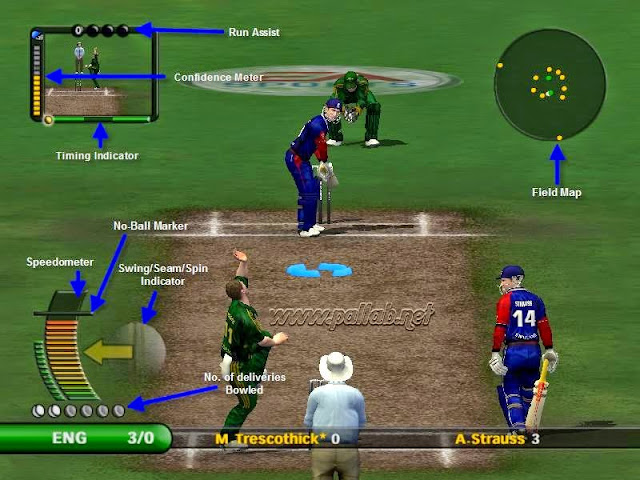
Molality (csucrose) = 0.1957 mol/0.033kg water = 5.93 m
Bomb itgraffiti movies & documentaries online. Elevation of BP = Kbp*msucrose = (0.5121 ˚C /m)(5.93m) = 3.03 ˚C
Boiling point of maple syrup is 100.0 + 3.03 = 103.03˚C
The boiling point stated above (taken from the producer’s websites) is 0.77˚ C higher than what we calculated. Ask the students to answer this question. Is this because, in reality, syrup also contains a small amount of other solutes that contribute to the increase in boiling point?
After boiling for some time we lose approximately 5 grams of water. There are still 67 grams of of sucrose in the solution. What is the boiling point now?
Molality (csucrose) = 0.19 mol/ 0.028 kg water = 6.99 m
Elevation of BP = Kbp*msucrose = (0.5121 ˚C /m)(6.99m) = 3.57˚C
Boiling point of maple syrup is 100.0 + 3.57= 103.57˚C
What is the percentage of maple sugar in the syrup now?
67g / 67g + 28g * 100 = 70%
References:
Download Urbackup Client
1. B. Z. Shakhashiri; Chemical Demonstrations: A Handbook for Teachers of Chemistry; Wisconsin; Volume 3; 1989; p. 283-285
2. J. Kotz, P. Treichel, J. Townsend; Chemistry & Chemical Reactivity; 7th Ed; Teachers Edition; Brooks/Cole; 2009; p. 635-637
